Abstract
Background Multiple myeloma (MM) is a clonal plasma cell disorder found in the bone marrow that produces a monoclonal immunoglobulin (M-protein). With blood based electrophoretic assays such as serum protein electrophoresis (SPEP) the M-protein is monitored, but with limited sensitivity.
Minimal residual disease (MRD) negativity during remission correlates with prolonged progression free survival. Lack of sensitivity prevents MRD detection by conventional blood based assays. Bone marrow based assays such as next-generation sequencing (NGS) are highly sensitive in measuring MRD. However, bone marrow biopsies introduce a risk of non-representative sampling and are invasive, which limits repeated testing.
Frequent MM monitoring during remission could provide actionable information on disease activity and treatment response. Earlier detection of disease progression could lead to early intervention and, potentially, patient survival benefits.
Aim The aim of this study was to perform M-protein monitoring on blood samples of MM patients with sensitive targeted mass spectrometry (MS-MRD), to show the capabilities of this MRD-approach.
Methods Forty-one MM patients were selected from the IFM 2009 study (ClinicalTrials.gov number: NCT01191060) with 13-31 serum samples per patient collected longitudinally over 29-60 months follow-up. RNA sequences, NGS-MRD, and SPEP data were available to us. All patients were treated with lenalidomide, bortezomib, and dexamethasone, but only treatment arm B received autologous stem-cell transplantation (ASCT).
For each patient, 2 unique tryptic peptides from the M-protein were selected, 1 peptide was selected for patients with only light chain M-protein. For early increases in disease activity, absolute M-protein quantification (g/dL) was performed based on tryptic peptides and stable isotope-labeled peptide as an internal standard.
Results Out of a total of 929 measured samples, M-protein was detected in 879 samples by MS-MRD and only in 195 samples by SPEP. Furthermore, MS-MRD showed a 78% concordance with NGS-MRD with 14 samples being MS-MRD positive but NGS-MRD negative. Sensitivity can vary per patient and was estimated between 0.03-0.57 mg/dL in this cohort.
Based on the M-protein profiles obtained with MS-MRD, during the maintenance treatment, increase of disease activity was observed in a subset of patients from arm A whereas all patients from arm B, who received ASCT, showed a persistent M-protein decrease.
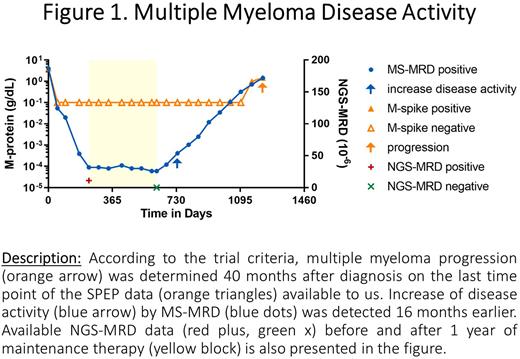
An increase of MM disease activity was observed earlier with MS-MRD in 13 out of 14 patients known to progress during the serum collection period, with a median of 455 days gained compared to the original trial data (p<0.0001). Figure 1 shows MS-MRD data showing disease activity 16 months prior to the known progression.
Conclusion A non-invasive and sensitive MS-MRD assay for frequent MM monitoring was implemented on clinical trial samples. MS-MRD showed that patients who received ASCT had a more durable response with decreasing M-protein levels. Moreover, in 93% of the cases an increase of disease activity was detected earlier than with conventional blood tests by more than a year.
Future perspective
The dynamic information on disease activity that is provided by the MS-MRD can provide patients with feedback on their disease status after each checkup, and can provide clinicians options for evidence based treatment escalation or de-escalation. While the overall patient benefit of such personalized treatment still needs to be assessed in clinical trials, we expect that this can improve patient quality of life and allow for more efficient use of pharmacotherapy.
Disclosures
Noori:Sebia (France, Lisses): Research Funding. Nadal:SEBIA: Current Employment. Stingl:Sebia (France): Research Funding. Luider:Sebia (France, Lisses): Patents & Royalties: Licensed Patent #19215329.4 to Sebia, Research Funding. Jacobs:Sebia (Lisses, France): Patents & Royalties: Licensed Patent #19215329.4 to Sebia, Research Funding. Vanduijn:Sebia (France, Lisses): Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal